Ringer 1.0 Users Manual
Authors:
P. Therese Lang
Ho-Leung Ng
Tom Alber
Copyright © 2010
Regents of the University of California
All rights reserved
Last updated August 2010
1.1. Why study side-chain dynamics?
Proteins populate structural ensembles that mediate folding, ligand binding, catalysis, signaling, and evolution. Recent developments in NMR spectroscopy have provided new insights into the relationship between structure, function, and dynamics. X-ray crystallography complements these techniques by providing detailed structural data on the nature of conformational polymorphism. Crystal structures are traditionally used to define protein dynamics by analyzing B-values, by detection of conformational differences in independent molecules within the same asymmetric unit, or independent crystal structures. In addition, proteins in crystals exchange hydrogens with solvent, undergo reversible conformational changes, and catalyze chemical reactions as complex as DNA polymerization. These and many other examples indicate that proteins in crystals can undergo small- and large-scale motions and populate diverse conformations.
In contrast to this evidence for conformational polymorphism, ~95% of residues in the crystal structures in the Protein Data Bank (PDB) are modeled by a single conformation. Crystal structures are typically built by interpreting electron density maps at 1 standard deviation (sigma) greater than the mean electron density of the unit cell. Although it represents a variable absolute electron density, this 1 sigma level is often considered to approximate the boundary between the signal from the molecule and the noise in the electron-density map. At high resolution, disorder can be modeled explicitly with the placement of alternate conformers into the electron density. To describe localized motions, atomic B factors represent a Gaussian decay of the electron density of the modeled atoms. To represent large-scale and collective motions, refinement may also include grouped anisotropic B factors. Because they combine contributions from motions, static disorder and model errors, however, B factors can disguise discretely disordered alternate side-chain and main-chain conformations.
To determine if electron density maps contain signals for small populations of alternate side-chain conformations, we developed a computational method, Ringer. Ringer automatically samples electron density around the side-chain dihedral angles of a structural model and identifies peaks that correlate with structural features. Using Ringer, we uncovered evidence suggesting unmodeled, alternate conformations in over 18% of side chains in a diverse set of 402 high-resolution structures. Evidence for small populations of alternate rotamers was also detected in an unusually accurate, experimentally phased electron density map, indicating that the signals from these low-occupancy conformers do not arise from phasing artifacts or model bias. Ringer also identifies changes in side-chain ensembles in high-resolution structures of calmodulin (CaM) in both an open state and bound to a peptide, suggesting these motions are related to folding and substrate recognition. These alternate structures provide a more dynamic picture of protein conformation and ligand binding. In general, these results indicate that systematic sampling of weak electron density features reveals conformational diversity in electron density maps that has been overlooked by traditional model building and refinement practices. These newly characterized conformations can be used to provide insights linking side-chain motions with protein function and drug binding.
1.2. What can Ringer do for you?
Ringer distinguishes flexible regions from rigid regions of biomolecules such as drug receptors. To assess the generality and significance of the weak secondary peaks of uniquely modeled residues, we ran Ringer on 402 high-resolution (<=1.5 Å) crystal structures from the Protein Data Bank. Omit electron-density maps were analyzed to reduce the effects of model bias. When applied after refinement is considered complete, Ringer discovers polymorphism at over 3.5 times the frequency that is currently modeled in the PDB. Multiple conformers are found for >18% of unbranched residues in a test set of 402 high-resolution structures, in addition to the 5.1% that are already modeled (1).
More than a method for enhancing crystallographic refinement, however, Ringer is best used as a tool for systematically detecting low-occupancy structural features. The hidden conformational substates identified using Ringer provide clues to the functional roles of protein structural polymorphism and to assess the response of protein side chain distributions to perturbations including ligand binding, temperature changes and mutations. In calmodulin, for example, Ringer identifies side chains that undergo conformational population inversions and side-chain rigidification upon peptide binding, linking the structure to dynamic properties (1). Similarly, in human proline isomerase, Ringer was used to define the nature of a coupled conformational switch in the free-enzyme that defines motions that occur during turnover (2) . In both cases, the alternate conformations identified by Ringer provided structural insights not available from any other experimental technique.
NOTE FOR NON-LINUX USERS: Ringer must be run using a Unix-like environment. For Macs, you will need to use the terminal window. For Windows, we typically use Cygwin (http://www.cygwin.com/) with a full Unix installation, including compilers and Unix shells.
1) Ringer is dependent on the external program Chimera (3). Go to the Chimera website (http://www.cgl.ucsf.edu/chimera/) and follow the installation instructions.
NOTE: Ringer has only been tested using the current release (version 1.4.1). If you use a different version and find problems, please contact us at ringer-users
lists.berkeley.edu with a description of the problem and the error message.
2) Save the distribution in the directory you want it installed in. Unpack the distribution using the following command:
[user@ringer ~] tar -zxvf ringer.tar.gz
3) Set the following environment variables: CHIMERA_HOME should point to the directory where Chimera is installed; RINGER should point to the directory where Ringer is installed. For example:
Linux Environment using tcsh
setenv CHIMERA_HOME /home/terry/programs/chimera
setenv RINGER /home/terry/bin/ringer-1.0Mac OS X Environment using bash
export CHIMERA_HOME=/Applications/Chimera.app/Contents/Resources
export RINGER=/Users/terry/ringer-1.0NOTE: For Mac Users, the CHIMERA_HOME variable needs to point to the Resources directory in the standard Chimera installation.
Windows (Cygwin) Environment using bash
export CHIMERA_HOME="/cygdrive/c/Program Files/Chimera/"
export RINGER='C:\Program Files\Cygwin\home\terry\ringer-1.0'NOTE: For Windows users, the CHIMERA_HOME variable needs to use the Cygwin-based path. The RINGER variable needs to use the MS-DOS-based path.
4) Test the installation
[user@ringer ~] cd $RINGER/test
[user@ringer ~] make
#The tests should complete in less than five minutes[user@ringer ~] make check
#This command will list any differences uncovered during testing.[user@ringer ~] make clean
#This command cleans up after the test is completeNOTE: Some failures are not significant. For example, differences in the tails of decimals may not be significant. The sources of such differences are frequently platform dependencies from computer hardware, operating systems, and compilers that impact arithmetic precision and random number generators.
The aim of Ringer is to go beyond static structural snapshots of proteins by uncovering structural ensembles in X-ray electron density. This information can reveal not only which parts of proteins are flexible and which part are rigid, but it also can define alternate conformations that may be important for function. Alternate conformations of binding sites also may afford additional targets for drug design.
|
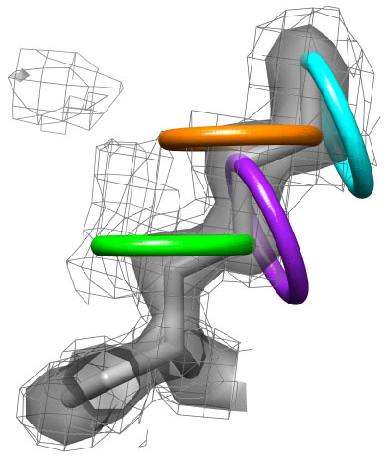
1) Ringer detects localized protein motions by sampling around the side chains. The backbone is held rigid.
|
2) The side chain motions are identified by a systematic search of X-ray electron density. Shown here is the electron density for Lys86 from
a putative tyrosine phosphatase (PDB ID 2HHG). The electron density is contoured at 1.0 sigma, which is the standard threshold when building a structure. |
3) Each side chain is sampled in rings by rotating the terminal heavy atom (gamma for chi 1) around the chi angle in user defined increments. |
4) At each sampled point, Ringer interpolates the electron-density value from the map. All electron-density values are considered, including contours below the standard 1.0 sigma cutoff. Shown here, for example, is the 0.3 sigma contour (mesh). |
5) The electron density values are plotted in two-dimensions against the sampled chi angles. |
6) Ringer then picks electron-density peaks from the plots. In this example, Ringer identifies all peaks above the 1.0 sigma cutoff (grey bar). A single peak (blue stars) is found for each chi angle, each of which corresponds to the built conformation. |
7) Ringer can also identify peaks below the standard cutoff. In this example, Ringer identifies an additional three peaks (red stars) above the 0.3 sigma contour (grey mesh)--evidence for additional, currently unmodeled conformations. |
Beyond Ringer: Ringer prints out the electron density vs chi angle plots as well as lists of peaks above a user-defined cutoff. With these data, the user can go back to the model and build in the alternate conformations. In this example, the secondary peak identified for the chi 1 angle (arrow) corresponds to an alternate conformation that has been been built into the electron density for the 0.3 sigma contour. |
|
Ringer must be run from the command line in a standard unix shell.
USAGE:
[users@ringer ~] $RINGER/ringer/ringer -i ringer.in [-o ringer.out]OPTIONS:
-i ringer.in #input file containing user-defined parameters
-o ringer.out #output file containing the parameters used in the calculation, summary information for each analyzed protein, and warning messages
Ringer analyzes crystallographic electron density using atomic coordinate files in standard PDB format. Electron density must be in the form of maps. The electron density maps can be computed from structure factors deposited in the PDB, downloaded from the Uppsala Electron Density Server, or calculated in the course of crystallographic refinement. Ringer reads electron density maps in CCP4, X-PLOR, and CNS file formats. The maps are expanded to ensure they cover the entire model. The sigma values in the map are scaled to ensure a mean electron density of 0 sigma and a standard deviation of 1 sigma. This scaling includes all voxels in the electron density, and it emulates the standard scaling in the commonly used modeling program, Coot.
Click here for a tutorial on how to use Ringer.
The parameter definitions will use the following format:
parameter_name [default] (value)
File Input
To find coordinate files, Ringer reads in a newline delimited list of PDB codes or file prefixes. To analyze a single pdb file, the list should contain only the single PDB code or file prefix.
pdb_list_file [None] (string)
If model filename or map filename has additional descriptors (eg refine005), they can also be defined. It is assumed that the file prefix is connected to the additional descriptor using an underscore.
pdb_name [None] (string)
map_name [None] (string)Finally, the absolute path to the model and map files must be defined.
pdb_file_location [./] (string)
map_file_location [./] (string)Side Chain Perception
Each chi angle is defined based on the modeled side chains. Chi 1 angles are built starting at the backbone nitrogen.
For residues with alternate conformations included in the model, Ringer either samples using the higher-occupancy conformation (off) or excludes the side chain from the analysis (on).
skip_multi_conf [on] (off,on)
Sampling Options
The bond length and angle used for moving the terminal atom is controlled by one of three options:
With all sampling options, dihedral angles that terminate in hydrogens are sampled with a standard sp3 carbon bond length of 1.53 Å and angle of 111.1°.
atom_sample_type [dynamic] (constant, experimental, dynamic)
The dihedral angles are systematically sampled by a changeable absolute torsion angle
chi_sample_degree [10] (integer)
The electron density at each point is extrapolated from the map electron density in Cartesian space by trilinear interpolation.
The electron density (in units of sigma) is plotted versus the sampled chi angles for peak identification. Peaks are identified as the maxima in the plots of sigma vs. chi angle above the user-defined lower cutoff in units of sigma.
lower_sigma_cutoff [0.3] (float)
Peaks also can be restricted below an upper threshold of 0.8 sigma. This filter helps to focus on peaks that represent low population conformations below the standard 1.0 sigma noise cutoff.
upper_sigma_cutoff [off] (on,off)
Verbose Output Options
The user has the option to write out various verbose portions of data from the calculation. The prefix of these files can be modified
verbose_outfile_prefix [ringer] (string)
The raw data to generate the sigma vs chi angle plots can be printed for amino acid types.
write_sigma_plot [off] (on,off)
plot_residue [LEU] (ALA, ARG, ASN, ASP, CYS, GLN, GLU, HIS, ILE, LEU, LYS, MET, PHE, SER, THR, TRP, TYR, VAL)Ringer can also print out the list of peaks identified from the sigma vs chi angle plots above the user defined cutoff. Because Ringer identifies alternate conformations based on density peaks beyond the primary peak, only unbranched torsion angles are chosen for each chi angle category. The identified peaks are divided into chi angle-based categories:
chi 1 = Ser, Gln, Asn, Glu, Asp, Arg, Lys, Met, Cys, and Leu
ring = Phe, Tyr, Trp, and His
chi 2 = Gln, Glu, Arg, Lys, Met, and Ile
chi 3 = Lys, Arg, and Met
chi 4 = Lys and Argwrite_peak_list [off] (on,off)
In reference 1, Arg, Asn, Asp, Gln, Glu, Leu, Lys , and Met residues were used for chi 1/ chi 2 “checkerboard” correlation analysis. The user has the option to print out the raw data used to generate this plot as well.
write_chi2chi1 [off] (on,off)
Peaks detected by Ringer currently require manual inspection and building of alternate conformers. Corroboration of individual alternate conformers should be obtained from Fo-Fc difference density, coupled backbone shifts, and connected electron density corresponding to the entire side chain. Non-rotameric peaks, which can arise from coupled main-chain shifts or high-energy conformations, can be difficult to build by visual inspection. We suggest using Ringer iteratively with model building and occupancy refinement. Side-chain conformations added to the model to fit Ringer peaks should be validated by small improvements in map quality and interpretability, real space correlation coefficients between Fc and Fobs, the consistency of B values or R/Rfree values.
1. Lang PT, Ng HL, Fraser JS, Corn JE, Echols N, Sales M, Holton JM, Alber T. Automated electron-density sampling reveals widespread conformational polymorphism in proteins. Protein Sci. 2010 Jul;19(7):1420-31.
2. Fraser JS, Clarkson MW, Degnan SC, Erion R, Kern D, Alber T. Hidden alternative structures of proline isomerase essential for catalysis. Nature 2009;462(7273):669-673.
3. Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera - A visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25(13):1605-1612.
4. Cornell WD, Cieplak P, Bayly CI, Gould IR, Merz KM, Ferguson DM, Spellmeyer DC, Fox T, Caldwell JW, Kollman PA. A second generation force field for the simulation of proteins, nucleic acids, and organic molecules. J Am Chem Soc 1995;117:5179–5197.